Historically there has been under-representation of ethnic and racial minorities in clinical trials the reasons are varied from low levels of trust in medical researchers, language barriers, levels of awareness and limited access to trials but also historically a low level of awareness amongst pharmaceutical companies and others for the need to have diverse cohort of patients.
Given that we are currently living through a Global COVID – 19 Pandemic, which has highlighted yet again the disproportionate effects on people from racial and ethnic minorities it is the right time to revisit diversity in clinical trials. This has urgency in reinforcing the important role that vaccines have played in protecting us.
Why is Diversity important in clinical trials? It Is not possible to secure a robust and more representative body of clinical knowledge to deepen our understanding of ethnic and racial differences in treatment responses without having a diverse cohort of participants. In addition to clinical knowledge, it is also a matter of ethics and equity.
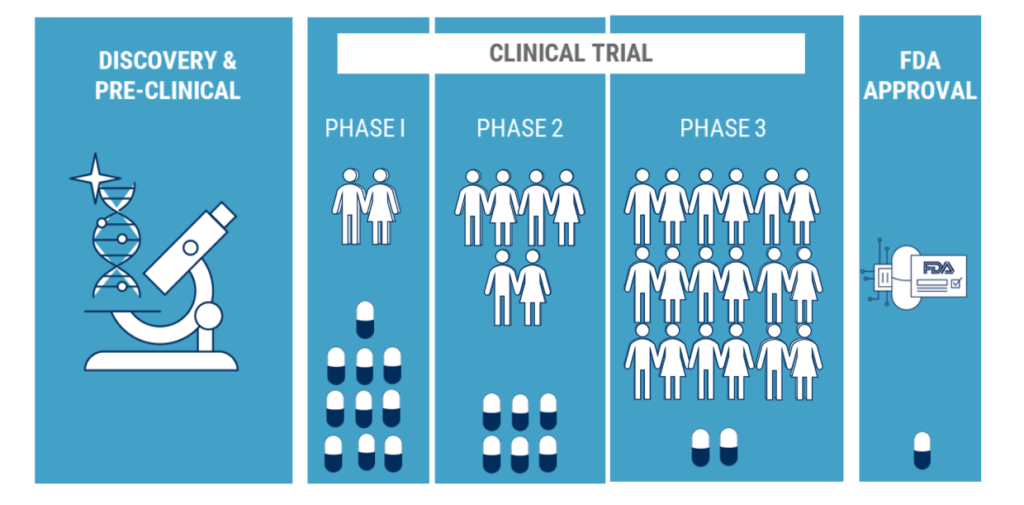
There have been small steps towards greater inclusion, four of the major pharmaceutical companies in the US have made pledges to ensure that their clinical trials will be inclusive. It is early to judge whether these pledges are being realised. What are the facts:
With all the debates around clinical trials and the importance of diversity in clinical research, such inclusion is not yet guaranteed. In an article in The Lancet June 2020, A systemic review published found that of 1,518- COVID-19 studies registered on ClinicalTrials.gov only six were collecting data on ethnicity.
In the UK there are no obligations to record and report ethnicity in clinical research studies.
The prevalence of Type 2 Diabetes in the UK, which disproportionally effects racial and ethnic minorities whose mean involvement in clinical trials was found to be 5.5%, despite one ethnic group representing 11.2% of the UK Type 2 Diabetes population. This brings into question the role of medical charities in the UK and their performance on issues of equity. Clearly a lot more needs to be done.
What next?
- Clearly Pharma companies want to improve health outcomes for all patients and all patients want to access the best treatment for their condition. A good start would be to begin with the design, methodology, criteria and enrollment practices of clinical trials that involve a broader range of stakeholders;
- Have strong partnerships to improve collaboration and work together to diversify the pool of patients involved in clinical research- without this the Industry will not achieve diverse patient centeredness;
- Education and Information for racial and ethnic minorities on the need to come forward and participate in clinical trials, highlighting benefits to them and the community;
- Making it mandatory that ethnicity and other population data be collected as part of every clinical trial and is made public;
- Reviewing the evidence on barriers to clinical participation and addressing them either through advanced technologies and or recruitment of researchers that are culturally competent;
- Community focused projects through Primary Care Networks or through other stakeholders [with high levels of trust] to build a Bank of diverse patients who have consented to being approached for clinical trials;
- Any public investment into health must mandate inclusion and diversity outcomes to be made publicly available, this should also apply to Charities in the UK that benefit not just from public investment but also from substantial tax advantages, with little accountability on equity.
- Organisations engaged in patient recruitment need to be able to demonstrate capacity and experience of recruiting ethnic and racial minorities as a condition of contract
Conclusion
The future of clinical trials is that they will be run by automation and Artificial Intelligence -big data analytics will play a huge part, it is critical that big data has been accurately capturing ethnicity and other population data. Without this in place AI will damage health equity further.
The urgent need for alliances to create a wider debate and influence both the political and Pharma landscape to embed and demonstrate diversity and inclusion in the clinical trials -without which winning the trust of the population on vaccines will remain a huge challenge.


Tony Kelly
April 26, 2021 at 3:22 pm
This is a well constructed article. New to this site so kindly explain why it is uncategorized?